What Causes Farmers to Return to the Slopes of Volcano to Begin Growing Crops Again
Introduction
Indonesia has more than 100 active volcanoes, and many more that are dormant, as it is function of the global ring of burn down linked to tectonic plate subduction zones. Densely populated Coffee and Bali reflect both the long-term soil fertility benefits of volcanic ash and a large number of people at chance during fresh eruptions, ash degradation, and lahar flows (Van Ranst et al., 2004; Achmad and Hadi, 2015). Volcanoes are regularly resetting the clock on vegetation succession and create a demand for "restoration" when downstream impacts such as mudflows of unconsolidated ash is to be controlled (van Noordwijk et al., 2020). Beyond scenic dazzler, volcanoes are also home to a limited, just specialized flora, contributing to the overall biodiversity of the country. The short-term damage of eruptions involves settlements, access roads, agricultural lands, agroforestry, and watershed protection forests, and calls for remedial disaster responses. Human resilience is challenged by damaged infrastructure, loss of boundaries of land ownership, destruction of forests, disturbed water catchment areas, and springs (Rahayu et al., 2014). Can a resilient local tree, adapted to volcanic ash environments be of assist? Current emergency preparedness documents in Indonesia are not enlightened of whatever such tree.
Atmospheric deposits after a volcanic eruption can consist of ash, sand, gravel, or stones with the lighter materials traveling further. Ash deposits modify the concrete, chemic, and biological properties of the soil surface (Achmad and Hadi, 2015). Thick layers of ash tin cause a dense, cement-similar soil surface afterwards rainfall (Suriadikarta et al., 2010). Emergent hydrophobicity decreases infiltration beneath what is expected given the substrate'south porosity and leads to a dry soil surroundings (Rudianto et al., 2017), with depression levels of organic C and N and a soil pH that can be acidic, neutral, or alkaline depending on SiO2 levels in the volcano'southward substrate (McGeary et al., 2002). Regrowth of vegetation is hindered past a challenging soil surround (Sinaga et al., 2015), but also by a lack of viable seed supply, an absence of biological dispersal agents and shelter for seedlings; regrowth from stumps is possible, withal, outside of the chief degradation zones. Lack of vegetation regrowth leads to the high mobility of the ash within the landscape and accumulations in the riverbeds, causing farther problems downstream, merely also opportunities for collecting "volcanic sands" that are a preferred resource for the building industry and allow former farmers a temporary source of income. Rapid recovery of vegetation, especially on the higher slopes is desirable to support the recovery of the hamlet economic system, notwithstanding.
The Kelud eruption (Due east Java, besides commonly spelled as Kelut) on 13 Feb 2014 was i in a long series of recorded eruptions and ash deposits with return times of xv–37 years (with ash deposits in intervening years every bit well): 1826, 1848, 1864, 1901, 1919, 1951, 1966, 1990 (Thouret et al., 1998) and a relatively minor event in 2007. These relatively short render times may exist function of the local option of species that chop-chop recover. In 2014 the top of the plume reached to a height of well-nigh 30 km and the umbrella deject spread radially at 17−20 km high; ash was recorded up to Bogor, West Coffee; due to the prevailing wind, there were big deposits on the north and northeast area of the volcano (Suzuki et al., 2014; Kristiansen et al., 2015). The 2014 eruption deposited well-nigh 50 × x6 one thousand3 of material on the upper slope of Kelud Volcano. A considerable office of this was washed down by rainfall in mudflows along the rivers and deposited downstream where the riverbed widened (Dibyosaputro et al., 2015). This was not the commencement time this happened, and function of the vegetation may be adjusted to these circumstances. The German explorer Junghuhn had in one,844 collected a tree specimen on Mt Kelud from which Miquel (1859) described the species as belonging to the genus Parasponia, equally it closely resembled Sponia, at present known as Trema. Local names confirm the close resemblance, with Trema known as "anggrung", and Parasponia as its greener sibling "anggrung hijau". Clason (1935) exploring the vegetation of the mountain slopes and valleys afterwards the Mount Kelud eruption of 1919, reported Parasponia particularly from the volcanic ash and lahar valley, while Trema grew more frequently in places where the original soil, "although probably more or less sterilized" had persisted after the eruption. Clason (1935) mentioned in passing that "Parasponia possesses root nodules, nitrogenous nutrient being maybe obtained in this way so that Parasponia is thus adapted as a pioneer type to virgin soil." Nevertheless, it took another xl years earlier this observation was noticed, although Smiet (1992) described Parasponia as a common part of Java's mountain flora. Trinick (1973), Akkermans et al. (1978), Trinick (1979), and Becking (1979) established the exception to the rule that only the Leguminosaeast tin associate with rhizobium bacteria. The genus Parasponia (in the Cannabaceae family, formerly seen as part of the Ulmaceae) that is known from volcanic ash environments in Republic of indonesia and the Philippines tin can form effective nodules with rhizobium leaner (Bradyrhizobium species co-ordinate to Trinick and Hadobas, 1989). Due to its more open canopy, Parasponia stands permit the development of a dumbo layer of the grass Saccharum, which in turns prevents other late-successional trees to establish. "It would seem that the Parasponia-socion has already attained its greatest evolution and that it does not hold its own; in whatsoever example, I saw very few young trees and on the other mitt several old trees which had died off. It seems as if Parasponia became established earlier the edaphic conditions allowed the development of the Saccharum-socion." This description suggests that Parasponia occupies the "regeneration niche" (Grubb, 1977; Pickett and White, 2013), being able to establish itself quickly in the farthermost conditions that prevail afterward a recent ash degradation consequence (given the short return period of eruptions), but by enriching the ash deposits with nitrogen, paves the manner for grasses to take over, which in turn delay succession to other woody vegetation. A deeper understanding of these ecological relations in the field and aspects of the "regeneration niche" is warranted, as the unique volcanic ash environment in which Parasponia is evolving may account for its unique properties.
A productive line of research has explored the molecular biology of Parasponia-rhizobium interactions and the evolutionary interpretation of such interactions as either independently (re)discovered in multiple plant families or lost from a large number of constitute families that are otherwise related to both rhizobium hosts (Geurts et al., 2012; van Velzen et al., 2018). While many Leguminosae are not only able to initiate N2-fixation past rhizobium only also down-regulate information technology when there is sufficient nitrogen in its internal circulation organisation, there still is debate well-nigh the ability of Parasponia to practise the same in laboratory test weather (Vassey et al., 2005; Op den Army camp et al., 2012; Yulia, 2013). Dupin et al. (2020) recently showed that in lab weather condition exogenous fixed-nitrogen inhibits nodulation on P. andersonii. Much less is known on the ecology of the species in its native surround. From the existing evidence and literature, information technology appears that Parasponia evolved in the specific "regeneration niche" of volcanic ash where access to a nitrogen source is essential for early establishment but may pave the way, by enriching the soil, for more competitive non-nodulating sibling (or unrelated) species.
The specific research questions for our exploration of vegetation on Mount Kelud later on the 2014 eruption were:
1) Has the about recent eruption had different effects on the populations of Trema and Parasponia? We quantified the composition of the recovering vegetation across the slope, with specific attention to the relative share of the two sibling species.
2) Which aspects of landscape position were related to biomass recovery? We compared seedling, sapling, pole, tree populations and biomass estimates with soil properties in toposequences at iii pinnacle zones
3) Is (constructive) nodulation of P. rigida in the regrowth phase related to soil nitrogen levels? Nosotros tested the hypothesis that downwards-regulation of N2 fixation is absent in this early phase of an evolving symbiosis with Rhizobium, leading to soil enrichment.
In relation to options for enhancing natural regeneration to restore landscapes, we will discuss the opportunities Parasponia provides to existing disaster preparedness plans for the area (and similar volcanoes elsewhere in Indonesia) to comprehend a more pro-active vegetation direction protecting seed sources in the highest zone.
Materials and Methods
Study Expanse
The research focused on the northeast side of Mount Kelud (Figure i), where virtually of the ash of the 2014 eruption was deposited. The satellite imagery for the area (Figure 1) compares the country cover before the eruption (peaking fourteen Feb 2014, BNPB, 2014) that covered an area at five–10 km from the crater with ash. Vegetation at a larger distance from the crater recovered, despite some tree bloodshed, while regrowth closer to the crater had to first from either seeds or stumps.
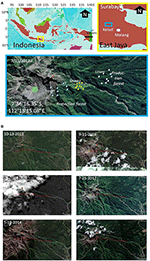
Effigy 1. (A) Location of Mount Kelud (Eastward Java, Indonesia), (B) with satellite imagery before (2012), directly after (March 2014), and in recovery phase from the 2014 eruption and the position of sampling points in lower, centre and upper elevational zone; the ruddy line indicates a five km transect.
The main access to the mount slopes we used was via the Kutut Pandansari village (7°54'35.74"S, 112°22'0189"E) in the expanse downstream of the Selorejo reservoir in Ngantang commune (Malang Regency). A path toward the crater passes through three meridian zones: lower (700–800 m a.s.50.), eye (900–1,000 thousand a.south.l.), and upper (>1,000 m a.south.fifty.) (Figure 1). The steeper slopes of Mount Kelud are classified equally "watershed protection" woods, with lower parts classified as production woods, both managed by the Country Forest Company (Perum Perhutani).
The research activity was carried out in two stages: Stage i focused on vegetation assay one year after the eruption (July—December 2015), and Phase ii on Parasponia nodulation was carried out 2 years afterwards the eruption (July—Nov 2017), with some follow-upwards observations at afterwards dates.
Data Collection
Observation Plots for Vegetation Analysis
Observation plots were selected for exploring the distribution of pioneer plant vegetation in various mural positions on the slopes of Mount Kelud starting from Mahogany plantations in the lower zone (700–800 grand a.s.fifty.), mixed vegetation at the middle (900–ane,000 m a.southward.l.), and upper zones (>ane,000 k a.southward.l.) using a survey scheme of plants on the slopes of Kelud mount (Smiet, 1992). In each toposequence an E-West transect was sampled, with ridge (eight–15% slope), mid-slope (40–60% gradient), and valley (0–3% slope) positions. Stand density was determined within patches of vegetation, not randomly sampling the mural.
Starting 2 years afterwards the eruption, stem diameter and tree biomass were measured for P. rigida in various landscape positions: zones (upper, middle, and lower) and for a toposequence of ridge, slope, and valley, as before.
Soil Analysis
In stage ane, soil samples were nerveless from each observation plot from soil depths of 0–ten, 10–xx, and 20–30 cm, respectively; soil samples per layer were composited from 5 points per plot. Dried soil samples were used for the analysis of full organic carbon (Corg) using the Walkley and Blackness (1934) method, total nitrogen (Due northtot) with a Kjeldahl procedure (Bremner, 1960), pH(H2O), and soil texture as standard t the Chemical Laboratory, Soil Science Department, Faculty of Agriculture, Brawijaya University, Malang. Split samples were taken for the decision of the soil majority density (Hairiah et al., 2011).
In phase ii, soil samples were taken at l cm distance from the main stem of P. rigida at a depth of 0–10, 10–xx, and 20–xxx cm were taken and analyzed for Corg, Due northtot (Kjeldahl), and mineral NH4 and NO3 concentrations in extracted soil solution using a colorimetric menstruation-injection autoanalyzer.
Vegetation
Ascertainment of vegetation was carried out on all types of vegetation in various growth stages (seedlings, saplings, poles, and copse). Sample observation plots were nested inside a 100 × 20 m primary plot (Kusmana, 1997), with v plots of 20 × 20 m used to measure trees (D > 10 cm; D = diameter at 1.3 thousand above the basis, also known as bore at breast pinnacle) (trees), subplots of 10 m × 10 thousand used for poles (five < D <10 cm), sub-sub plots of v × v m for saplings (D < 5 cm, H > two m), and sub-sub-sub plots of ii × two grand for seedlings (H < ii grand).
Assessment of the Effectiveness of Root Nodules
Root nodules were sampled by sorting through all soil in a 50 × 50 × 10 cm depth sample adjacent to ten trees that were considered representative of the stand (avoiding the largest and smallest 20% of the distribution equally found in the stand). All root nodules were separated from the roots, counted, and stored in seventy% alcohol. All nodules were observed nether a dissecting microscope for hemoglobin color on a cantankerous-section. Red hemoglobin was taken equally an indicator of effectiveness, white nodules were classified equally non-effective (Sarasawati, 2007).
Additional fresh nodule samples and rhizosphere soil adherent to roots was also nerveless for quantification of rhizobium density, via a dilution series and plating on petri dishes kept at room temperature (around xx°C) for 7 days to estimate the number of Colony Forming Units (CFU). Details of the method are described past Sarasawati (2007). The (autoclaved at 250°C) growth medium consisted of Potato Dextrose Agar (7.8 g), Mannitol (2 g), Yeast excerpt (0.two thousand), G2HPO4 (0.1 g), MgSO4 (0.04 thousand), NaCl (0,1 g) in 200 ml distilled water, with Congo Red (0.25 g) added as indicator. The highest dilution that still showed bacterial colony formation was taken every bit an indicator of the rhizobium concentration in the original sample, with calculations specified in Sarasawati (2007).
Parasponia's Taxonomic Position
The GlobalTreeSearch (Beech et al., 2017) mentions only 1 Parasponia species for Indonesia P. rigida Merr. & Perry (Hassler, 2019). In the Kew Garden plant list, all species in the genus Parasponia are indicated as "unresolved." Role of the literature on Indonesia [including Ishaq et al. (2020) refers to P. andersonii Planch. which has been confirmed for islands in the Pacific. Role of contempo taxonomic interpretation places the species into the genus Trema (which has Sponia, to which Parasponia refers, as a synonym), every bit T. rigida (Merr. & 50.G. Perry) Byng & Christenh. Pending this taxonomic debate, we will here utilise P. rigida as the botanically correct proper noun for the tree species plant on Mount Kelud.
Information Analyses
Pedotransfer as Reference Values for Soil Parameters
A reference value Cref for the Corg concentration expected under long-term forest conditions for a soil of the same texture and pH at the same elevation (with additional factors for Andisol and Wetland conditions) was used, based on van Noordwijk et al. (1998) and Hairiah et al. (2020), including a depth correction for a soil layer from ZH to ZFifty cm depth:
with elevation expressed in m a.s.fifty. and "Andisol?" and "Wetland?" are zero unless the specific soil condition (with higher Corg) applies and the value is 1.
Vegetation Analysis
The diversity of vegetation was characterized by identifying all woody plants in the observation plots and calculating several indices from the results: density (number of individuals per unit area), frequency (fraction of plots in which a species was found), dominance (relative abundance) and the INP or Index of Importance Values (Soerianegara and Indrawan, 1978).
with basal area (BA) derived equally Σ π DBHtwo/iv from measurements of DBH or stem bore at breast height (i.three m).
Diversity Index (Shannon and Wiener, 1949):
where H′ = Diverseness index, N = Full number of individuals sampled, ni = Number of species i.
Evenness index (with values between 0 and 1):
where Pi are the frequencies of the grand species observed.
Tree Biomass
Measured stem diameters of poles and trees (for D > 5 cm) were converted to aboveground biomass (AGB) estimates using allometric equations for wet tropics (boilerplate rainfall one,500–four,000 mm y−1) from Chave et al. (2005):
Where ρ = wood density (chiliad cm−3), derived from http://db.worldagroforestry.org/wd. A locally developed (Alfian, 2017) species-specific allometric equation was used: AGB = 0.4992 Dane.0012.
Statistical Analysis
The results of observations and measurements of biophysical data were analyzed by Analysis of Variance using Genstat (18th edition) software to test possible rejection of a null-hypothesis of no furnishings of toposequence and landscape positions every bit main factors, along with a uncomplicated interaction term. Where statistically meaning differences (p < 0.05) were noted, a Duncan exam was used to compare means at the combination of the two factors. Correlation and linear regression analysis (in the MS-Excel software) were used to depict relationships between response (Y) and explanatory (Ten) parameters, as Y = b Yg + (a Ygrand /10thou) 10, afterwards normalizing or rescaling data sets relative to their means, Ym and Xg, respectively.
Results
Vegetation and Soil Properties
Soil profiles in the surface area showed bear witness of multiple ash deposition events, with topsoils buried by the xiv February 2014 and earlier Kelud eruptions at various depths (Figure 2). Soil analysis (Tabular array 1) showed buried topsoil (with relatively high Corg) in the xx–30 cm depth layer, and slightly college pH values. Lower elevations had higher silt content, but the sand fraction dominated in all samples.

Effigy two. Soil profile on the ridge position at three elevations, with soil layers as identified in the field indicated by dashed lines and topsoil contempo ash deposits of close to 20, 12, and 7 cm, respectively; the measuring tape indicates depth increments of 10 cm (Photograph courtesy from Nugraha, 2015).
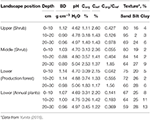
Tabular array 1. Soil properties for three soil layers in various mural positions (BD, bulk density; Cref, reference Corg concentration based on pedotransfer function; averages for three replicate plots).
Most of P. rigida plants were constitute in clusters on sites with a slope of 30–80%, spread over an altitude of 600–1,700 m a.s.50. at the lower, eye, and upper mural positions, with sandy and relatively moisture soil conditions. Usually, P. rigida was found in open shrubland, but it also developed well in the lava menstruation deposits (layers of sand and gravel) in the riverbeds (Figure 3D).

Effigy 3. P. rigida (dashed yellow lines) stands at various landscape locations: (A) Caldera, (B,C) high ridges, (D) Ash deposits in a river valley (Photo credits: start author).
Basal area, biomass, and necromass of trees differed significantly between landscape positions (Table 2). Necromass was 34 and 26% of biomass at upper and centre landscape positions, indicative of eruption-related tree mortality, but no necromass was observed in the lower zone, while biomass in that location was highest. In the centre and upper zones, several trees had survived, as indicated by the biomass of trees to a higher place thirty cm bore. Delight note that the vegetation sampling referred to existing stands, non to their frequency within the wider landscape. The absenteeism of necromass at lower peak can exist an indication of firewood collection by the neighboring hamlet.

Table 2. Tree biomass and necromass differentiated by stem diameter in stands in three mural positions (based on three replicates in each of 3 elevational zones).
Tree population data (Table three) matched the basal area (Tabular array 2) data, with the high biomass but low tree diversity (and well-nigh-absenteeism of internal regeneration) in the Mahogany stands in the lower zone). Tree diversity in the middle zone was significantly (p < 0.01) higher than that in the upper zone for trees (D > ten cm) and poles (v < D < 10 cm) but was not distinguishable between these ii zones for the sapling and seedling stages. In the latter two categories, however, sapling and seedling multifariousness reflected that in poles and trees at the high landscape position.

Table 3. Tree populations in various growth stages and biodiversity indices in 3 landscape positions (based on iii replicates in each of three elevational zones).
For further analysis of the differences between the zones, the Importance Value Index combines relative density, relative frequency, and relative domination for the commonest species. In the heart zone the seedling and sapling stage was dominated by Ageratum conyzoides (babadotan, locally called "Tropos") with INP = 177% and INP = 116%, respectively, and the pole stage past T. orientalis (INP = 61%). In the upper zone bulb and sapling, stages were dominated by Begonia multangular ("Mencok") with INP = 100% and INP = 63%, respectively, while the pole stage was dominated P. rigida and T. orientalis, both?? with INP = 226%.
Biomass Production and Root Nodule Density of P. rigida
Results for the 2d survey showed that for each of the three elevational zones, the P. rigida population density, as well as average stalk diameter, varied with position along the local toposequence (Figure iv). While population density was highest (80–200 trees/ha) in the valley positions in the upper and middle elevational zone, respectively. Tree bore was highest in the ridge. Please note that in this second survey the lower zone toposequence did not include the Mahogany plantation, just P. rigida numbers were relatively depression while other vegetation dominated.
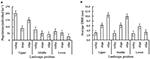
Figure 4. P. rigida operation in various toposequence and elevational positions on the slopes of Mount Kelud (three sample plots per height * mural position). (A) Total population of P. rigida with standard error of differences, (B) Boilerplate stem bore (DBH).
Plot-level biomass estimates (Figure 5A) bear witness by far the highest P. rigida biomass (164 kg plot−1) on the ridges in the upper zone. The relative share of P. rigida and T. orientalis in their combined tree population showed that T. orientalis was absent at the highest elevation and dominated at the middle elevation (Figure 5B).

Effigy 5. (A) Total biomass production of P. rigida (per xx × 20 mii plot) in the various landscape positions and elevation zones, with standard error of differences; (B) relative share of P. rigida and T. orientalis in their combined tree population.
Soil samples of the rhizosphere soil in existing P. rigida stands (Tabular array 4) showed domination by the sand-sized fraction (fresh ash), with silt and clay at slope and ridge positions in the centre and lower zone. Bulk density was high (1.2 g cm−3 to 1.six g cm−3, Ntot (0.012–0.040%), and Corg (0.13 to 0.26%) concentrations very low in all stands.
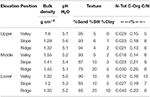
Table iv. Characteristics of soil physico-chemical properties in the P. rigida rhizosphere (three replicates).
Based on the hemoglobin color assessment 79–93% of nodules were classified equally "effective," while nodule densities per m2 of soil surface were highest in the valley positions at low and middle zone, and in the ridge position in the upper zone (Table 5).
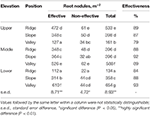
Tabular array 5. Root nodules of P. rigida in various locations, distinguished by effectiveness (hemoglobin color) (averages for 3 replicates).
Regression analysis of nodules on the iii indicators of soil nitrogen supply in P. rigida stands, Ntot, mineral and , was based on normalized parameters to let direct comparison between these indicators. The regression of the density of effective nodules on soil nitrogen indicators accounted for at least 85% of the observed variation (Figure half dozen). Nodulation was nigh abundant and effective on the poorest sites.

Figure 6. Relationship of effective root nodules with total Due north-content of the soil, with both X and Y variates normalized and expressed relative to the mean; (A) nodules per unit soil area vs. Ntot; (B) nodules per unit of measurement soil volume vs. Due northtot, nitrate and ammonium levels in soil solution.
Discussion
We found P. rigida populations over a considerable elevational range, from 600 to 1,700 m a.s.l., on around Mount Kelud later the recent eruption. A comparison of satellite imagery before (2013) and later on (2014) the eruption (Nuzulah, 2016) indicated a decrease in vegetation density up to a radius of 10 km from the caldera, with a vegetation density index decreasing from 0.64 to 0.34 in 2013 to 0.52–0.01 in 2014; despite a partial recovery in 2016, vegetation was afflicted until a radius of 5 km. The observations of authorization past P. rigida in the highest zone (>1,000 1000 a.s.fifty.) demonstrate the remarkable adaptation of this species to the extreme environs of frequently erupting volcanoes and abundant ash degradation. Similar observations in 2018 in well-nigh 2 km from the active crater of Mount Merapi (Central Java) subsequently an eruption in 2010 showed low levels of constitute diverseness, but P. rigida was observed in dense monospecific stands with some patches of Acacia decurrens (Dr. Subekti Rahayu, pers. comm. 2020). According to local informants on Mountain Merapi P. rigida only occurred on the ridge and along riverbanks before the contempo eruption, merely it spread out throughout the landscape afterward the eruption. The literature on the vegetation of Mount Kelud in the past and the high frequency of eruptions match an interpretation that the species combines the effective colonization of T. orientalis (Mangopang, 2016) a pantropical pioneer species with the ability to thrive on soils of very low nitrogen content. The distribution of P. rigida observed suggests that its seeds may exist carried past overland and river flows from trees on higher ridges, including the slopes of the caldera itself. P. rigida appeared to develop well in nutrient-poor and open soil conditions where the plants produce root nodules and the fresh greenish of leaves (Figure three).
Our observations advise that the stands on ridges in the highest zone (with populations of around 150 trees ha−1) had the highest biomass and were the likely seed source for abundant regeneration in the valley positions at the heart and lower elevation. Seeds can exist produced within 1 twelvemonth of a regrowing stand. The trees also contribute to soil formation, with a half-life time of litter of around twenty weeks (Ishaq et al., 2020). The sibling species T. orientalis and P. rigida co-occurred at lower and middle top, while only P. rigida was found in the highest zone. These findings suggest that the selective advantage of the nodulated P. rigida over its non-nodulated sibling species T. orientalis is most pronounced in the most extreme and N-poor parts of the landscape. In the lusher vegetation at lower elevations, T. orientalis can maintain a presence among grasses and other trees, P. rigida is abundant as a pioneer on the ash deposits in the valley merely appears to lose out from other vegetation later in the succession. From the absence, at this stage of the recovery afterward the most recent eruption, of T. orientalis in the highest zone we cannot distinguish between lack of seed sources or lack of power to grow as direct explanation, while P. rigida clearly meets both requirements for restoration success. Successful seed sources that can reach the rest of the mural, notwithstanding, do depend on the ability of pioneer plants to grow in the relatively harsh and nitrogen-poor soil weather. The reduced role of P. rigida across the pioneer zone may suggest that the ability of Parasponia species to nodulate has had negative consequences, relative to their Trema siblings, for competitiveness under less farthermost soil conditions. From these observations, it appears that explanations of the distributions of P. rigida and T. on the slopes of Mount Kelud will consist of the position of seed sources that survive the eruption, supported by the successful colonization of N-poor substrates by P. rigida. In more sheltered and Due north-rich mural positions, T. orientalis appears to have an edge in growth rates—but a direct test of competitive power across levels of soil Northward availability has yet to be performed. Styger et al. (2009) reported from secondary forest regeneration sites in Madagascar that 3-years erstwhile T. orientalis had a biomass of eight.5 Mg ha−1 and 5-years old stands 24.7 Mg ha−1. These biomass information, in a less extreme environment, are higher than what T. orientalis and P. rigida achieved on Mt Kelud, but effective soil embrace was achieved and ash deposits along the riverbed were stabilized past P. rigida seedlings.
Our findings of a negative response of nodule formation to external nitrogen supply in the field match the laboratory results reported recently by Dupin et al. (2020). The density of effective nodules per unit soil surface expanse was associated with below-average soil nitrogen indicators, with rhizobium populations in the nodules (153 × 104 to 112 × 106 CFU yard−1) up to a 100-fold increase higher up their concentrations in rhizosphere soil (average 82 × ten4 CFU g−i). For comparison, Widawati (2015) reported a rhizobium density in the legume kaliandra (Calliandra tetragona) nodules of ii.2 × tenvi CFU g−i.
Current emergency management plans for agile volcanoes like Mount Kelud rely on nursery-produced legume trees as planting textile to stabilize ash and reduce downstream mudflow risks. Three years afterwards the eruption of Mount Kelud other surveys in the restoration surface area (Tanjungsari et al., 2018) found the introduced legume tree Calliandra [both the crimson (C. tetragona calothyrsus) and white (C. tetragona) species to accept been planted, along with naturally dispersed mahang (Macaranga hispida), and anggrung (T. orientalis)]. Relative to such plans and exercise, our findings of a key role for P. rigida stands on the ridges at high elevation are highly relevant. These trees tin can recover rapidly after an eruption and probably are the main seed source for successful natural regeneration of tree-based vegetation in the mail service-eruption mural. P. rigida is not only a very interesting biological object of study, suggesting the weather condition nether which association between higher plants and rhizobium leaner can evolve, information technology also deserves a key role in emergency preparedness plans for the expanse. Some investment in securing local P. rigida seed sources for assisted seed rains could likely speed upwards the recovery procedure in next steps of the eruption cycle.
Conclusions
i. P. rigida populations survived the contempo eruption of Mount Kelud at an elevation of 600–one,700 one thousand a.south.50. The sibling species T. orientalis and P. rigida (differing in their ability to nodulate) co-occurred at lower and middle elevation, while only P. rigida was establish in the highest zone.
ii. The rate of biomass recovery was related to landscape position: Stands on ridges in the highest zone (around 150 copse ha−one) had the highest biomass and were the likely seed source for abundant regeneration in the valley positions at center and lower elevation.
three. The density of effective nodules per unit soil surface surface area was associated with beneath-average soil nitrogen indicators.
4. A practical implication of these findings is that existing P. rigida stands on the ridges at high elevation are cardinal to the successful natural regeneration of tree-based vegetation in the post-eruption landscape and deserve protection and a central function in emergency preparedness plans for the area.
Data Availability Statement
The raw data supporting the conclusions of this article volition exist made bachelor by the authors, without undue reservation.
Author Contributions
The field and laboratory research was carried out by RM and IA as part of their Main of Science plan at Brawijaya University, nether direct supervision of KH. RM and IA nerveless and analyzed the data. RM, KH, and MN designed the research and drafted the manuscript, for which all authors agree to be accountable for the content of the work. All authors contributed to the article and canonical the submitted version.
Funding
We admit funding from BOPTN 2015 (State academy operational assistance) of Brawijaya Academy in 2015 and cooperation with farmers of Kutut and Pandansari village, Ngantang sub-commune (East Java Province) that were affected by the Mountain Kelud eruption, for their helped to behave out this research and for their kindness to share their ecological knowledge of the area. Publication was financially supported by the WCU program /UN10/PN/2020.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential conflict of interest.
The reviewer RG declared a shared affiliation, with no collaboration, with one of the authors, MN, to the handling editor.
Acknowledgments
We thank Dr. Rene Geurts (Wageningen University) for putting us on the Parasponia trail. Nosotros acknowledge cooperation with the people of Kutut and Pandansari village who, despite being affected by the Mount Kelud eruption, assisted with the enquiry and shared their ecological knowledge of the area.
References
Achmad, S. R., and Hadi, H. (2015). Identifikasi sifat kimia abu vulkanik dan upaya pemulihan tanaman karet terdampak letusan gunung kelud. Studi kasus kebun ngrangkah pawon jawa timur. Balai Penelitian GETAS. Salatiga. Warta Perkaretan. 34, nineteen–30. doi: 10.22302/ppk.wp.v34i1.lx
CrossRef Full Text | Google Scholar
Akkermans, A. D. Fifty., Abdulkadir, S., and Trinick, M. J. (1978). N2-fixing root nodules in ulmaceae: parasponia or (and) trema spp? Plant Soil 49, 711–715. doi: 10.1007/BF02183301
CrossRef Full Text | Google Scholar
Alfian, I. (2017). Reklamasi Lahan Pasca Erupsi Gunung Kelud Secara Biologi: Evaluasi Ketersediaan N Dalam Tanah dan Kerapatan Bintil Akar Parasponia Andersonii. Skripsi. Malang: Universitas Brawijaya.
Google Scholar
Becking, J. H. (1979). Root-nodule symbiosis between rhizobium and parasponia (Ulmaceae). Plant Soil 51, 289–296. doi: 10.1007/BF02232892
CrossRef Full Text | Google Scholar
Beech, E., Rivers, M., Oldfield, S., and Smith, P. P. (2017). GlobalTreeSearch: the showtime complete global database of tree species and country distributions. J. Sustain. For. 36, 454–489. doi: ten.1080/10549811.2017.1310049
CrossRef Full Text | Google Scholar
Bremner, J. M. (1960). Determination of nitrogen in soil by the kjeldahl method. J. Agric. Sci. 55, 11–33. doi: 10.1017/S0021859600021572
CrossRef Full Text | Google Scholar
Chave, J., Andalo, C., Brown, S., Cairns, M. A., Chambers, J. Q., Eamus, D., et al. (2005). Tree allometry and improved interpretation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99. doi: ten.1007/s00442-005-0100-10
PubMed Abstract | CrossRef Full Text | Google Scholar
Clason, E. W. (1935). The vegetation of the upper-badak region of mountain kelut [East Java]. Bull. Jard. Bot. Buitenzorg xiii, 509–518.
Google Scholar
Dibyosaputro, S., Dipayana, Chiliad. A., Nugraha, H., Pratiwi, M., and Valeda, H. P. (2015). Lahar at kali konto after the 2014 eruption of kelud volcano, east coffee: impacts and adventure. Forum Geografi 29, 59–72. doi: 10.23917/forgeo.v29i1.793
CrossRef Full Text | Google Scholar
Dupin, S. E., Geurts, R., and Kiers, Eastward. T. (2020). The non-legume Parasponia andersonii mediates the fitness of nitrogen-fixing rhizobial symbionts under loftier nitrogen conditions. Front. Institute Sci. 10:1779. doi: 10.3389/fpls.2019.01779
PubMed Abstract | CrossRef Full Text | Google Scholar
Geurts, R., Lillo, A., and Bisseling, T. (2012). Exploiting an ancient signalling machinery to relish a nitrogen fixing symbiosis. Curr. Opin. Establish Biol. 15, 438–443. doi: ten.1016/j.pbi.2012.04.004
PubMed Abstract | CrossRef Full Text | Google Scholar
Grubb, P. J. (1977). The maintenance of species-richness found communities: the importance of the regeneration niche. Biol. Rev. 52, 107–145. doi: 10.1111/j.1469-185X.1977.tb01347.10
CrossRef Full Text | Google Scholar
Hairiah, G., Dewi, Due south., Agus, F., Velarde, S. J., Ekadinata, A., Rahayu, S., and van Noordwijk, M. (2011). Measuring Carbon Stocks Across State Employ Systems: A Transmission. Bogor: World Agroforestry Center (ICRAF) Southeast Asia Regional Program, 154.
Google Scholar
Hairiah, G., van Noordwijk, M., Sari, R. R., Saputra, D. D., Suprayogo, D., Kurniawan, S., et al. (2020). Soil carbon stocks in indonesian (agro) forest transitions: compaction conceals lower carbon concentrations in standard accounting. Agric. Ecosyst. Environ. 294:106879. doi: x.1016/j.agee.2020.106879
CrossRef Full Text | Google Scholar
Hassler, Chiliad. (2019). "Parasponia rigida," in World Plants: Synonymic Checklists of the Vascular Plants of the Earth, eds. Y. Roskov, L. Abucay, T. Orrell, D. Nicolson, N. Bailly, P. Kirk et al. Available online at: http://www.catalogueoflife.org/ (accessed May 3, 2020).
Google Scholar
Ishaq, R. Yard., Saputra, D. D., Sari, R. R., Suprayogo, D., Widianto, Westward., Prayogo, C., et al. (2020). Turning volcanic ash into fertile soil: farmers' options in java agroforestry after the 2014 mount kelud eruption. AGRIVITA. J. Agric. Sci. 42, 78–91. doi: ten.17503/agrivita.v42i1.2494
CrossRef Full Text | Google Scholar
Kristiansen, Due north. I., Prata, A. J., Stohl, A., and Carn, S. A. (2015). Stratospheric volcanic ash emissions from the 13 February 2014 kelut eruption. Geophys. Res. Lett. 42, 588–596. doi: 10.1002/2014GL062307
CrossRef Full Text | Google Scholar
Kusmana, C. (1997). Metode Survey Vegetasi. Bogor: Penerbit Institut Pertanian.
Google Scholar
Mangopang, A. D. (2016). Morfologi Trema orientalis (L.) Blume dan Manfaatnya Sebagai Tanaman Pionir Restorasi Tambang Nikel. Makasar: Prosiding Seminar Nasional from Basic Science to Comprehensive Educational activity.
Google Scholar
McGeary, D., Plummer, C. C., and Carlson, D. H. (2002). Physical Geology Earth Revealed. Boston: McGraw Hill Higher Educational activity, 574.
Google Scholar
Miquel, F. A. West. (1859). Flora van Nederlandsch Indië, Vol. 2. Amsterdam: Van der Postal service.
Google Scholar
Nuzulah, Due south. North. (2016). Kajian dinamika suksesi vegetasi di kawasan terdampak erupsi gunung api kelud berbasis information penginderaan Jauh Tahun 2013-2016. 17(1) Thesis, Report Program Geography, Concentration of Potential and Mapping Areas,Department of Geography, Kinesthesia of Social Sciences, Universitas Negeri Malang.
Google Scholar
Op den Campsite, R. H., Polone, E., Fedorova, Due east., Roelofsen, W., Squartini, A., Op den Camp, H. J., et al. (2012). Nonlegume Parasponia andersonii deploys a broad rhizobium host range strategy resulting in largely variable symbiotic effectiveness. Mol. Found Microbe Interact. 25, 954–63. doi: 10.1094/MPMI-xi-11-0304
PubMed Abstract | CrossRef Full Text | Google Scholar
Pickett, S. T., and White, P. S. (2013). The Ecology of Natural Disturbance and Patch Dynamics. Amsterdam: Elsevier.
Google Scholar
Rahayu, R., Ariyanto, D. P., Komariah, Chiliad., Hartati, South., Syamsiyah, J., and Dewi, Westward. S. (2014). Dampak erupsi gunung merapi terhadap lahan dan upaya-upaya pemulihannya [Impacts of the eruption of Mount Merapi on state and livelihoods]. Caraka Tani 29, 61–72. doi: x.20961/carakatani.v29i1.13320
CrossRef Full Text | Google Scholar
Rudianto, G., Indradewa, D., and Utami, S. N. H. (2017). The influence of volcanic ash thickness in the soil surface that fell diverse of growth phase to the growth and result of maize (Zea mays 50.). Vegetalika 6, 1–11. doi: ten.22146/veg.27959
CrossRef Full Text | Google Scholar
Sarasawati, R. (2007). Bakteri Pembentuk Bintil Akar dalam Metode Analisis Biologi Tanah. Bogor: Balai Penelitian dan Pengembangan Sumberdaya Lahan Pertanian, 27–38.
Google Scholar
Shannon, C. E., and Wiener, W. (1949). The Mathematical Theory of (USA) Communication: Unknown Altitude Part. Urbana, IL: Illinois Press.
Google Scholar
Smiet, A. C. (1992). Forest environmental on java. Homo affect and vegetation of montane forest. J. Trop. Ecol. 8, 129–152. doi: 10.1017/S026646740000626X
CrossRef Full Text | Google Scholar
Soerianegara, I., and Indrawan, A. (1978). Indonesian Forest Ecology. Bogor: Departemen Manajemen hutan, Fakultas Kehutanan Institut Pertanian Bogor.
Google Scholar
Styger, E., Fernandes, East. C. M., Rakotondramasy, H. M., and Rajaobelinirina, Eastward. (2009). Degrading uplands in the rainforest region of madagascar: fallow biomass, nutrient stocks, and soil food availability. Agrofor. Syst. 77, 107–122. doi: 10.1007/s10457-009-9225-y
CrossRef Full Text | Google Scholar
Suriadikarta, D. A., Id, A. A., Sutono, D. E., Santoso, East., and Kasno, A. (2010). Identifikasi sifat kimia abu volkan, tanah dan air di lokasi dampak letusan gunung Merapi [Chemical properties of volcanic ash erupted from Mountain Merapi]. Bogor: Balai Penelitian Tanah.
Google Scholar
Suzuki, Y., Iguchi, G., Maeno, F., Nakada, South., Hashimoto, A., Shimbori, T., and Ishii, One thousand. (2014). "3D numerical simulations of volcanic plumage and tephra dispersal: reconstruction of the 2014 kelud eruption," in AGU Fall Coming together Abstracts V53E-02 (San Francisco, CA). Bachelor online at: https://ui.adsabs.harvard.edu/abs/2014AGUFM.V53E.02S/abstruse (accessed November 24, 2020).
Google Scholar
Tanjungsari, A., Hakim, L., and Retnaningdyah. (2018). Provisioning and cultural services of restored ecosystem in mount kelud afterward 2014 eruption. J PAL 9:336. doi: 10.21776/ub.jpal.2018.009.02.01
CrossRef Full Text | Google Scholar
Thouret, J. C., Abdurachman, Grand. Eastward., Bourdier, J. L., and Bronto, S. (1998). Origin, characteristics, and behaviour of lahars post-obit the 1990 eruption of Kelud volcano, eastern Coffee (Indonesia). Bull. Volcanol. 59, 460–480. doi: 10.1007/s004450050204
CrossRef Total Text | Google Scholar
Trinick, M. J. (1973). Symbiosis between rhizobium and the non-legume, trema aspera. Nature 244, 459–460. doi: x.1038/244459a0
CrossRef Full Text | Google Scholar
Trinick, G. J., and Hadobas, P. A. (1989). Competition by Bradyrhizobium strains for nodulation of the nonlegume Parasponia andersonii. Appl. Environ. Microbiol. 55, 1242–1248. doi: 10.1128/AEM.55.five.1242-1248.1989
PubMed Abstruse | CrossRef Total Text | Google Scholar
van Noordwijk, M., Cerri, C., Woomer, P. Fifty., Nugroho, Chiliad., and Bernoux, M. (1998). Soil carbon dynamics in the humid tropical forest zone. Geoderma 79, 187–225.
Google Scholar
van Noordwijk, M., Ekadinata, A., Leimona, B., Catacutan, D., Martini, E., Tata, H. L., et al. (2020). "Agroforestry options for degraded landscapes in southeast Asia," in Agroforestry for Degraded Landscapes: Recent Advances and Emerging Challenges, eds. J. C. Dagar, S. R. Gupta and D. Teketay (Springer: Singapore), 307–347. doi: 10.1007/978-981-15-4136-0_11
CrossRef Full Text | Google Scholar
Van Ranst, East., Utami, S. R., Vanderdeelen, J., and Shamshuddin, J. (2004). Surface reactivity of Andisols on volcanic ash along the Sunda arc crossing Java Island, Republic of indonesia. Geoderma 123, 193–203. doi: 10.1016/j.geoderma.2004.02.005
CrossRef Full Text | Google Scholar
van Velzen, R., Holmer, R., Bu, F., Rutten, L., van Zeijl, A., Liu, West., et al. (2018). Comparative genomics of the nonlegume Parasponia reveals insights into evolution of nitrogen-fixing rhizobium symbioses. Proc. Natl. Acad. Sci. U.South.A. 115, E4700–E4709. doi: 10.1073/pnas.1721395115
PubMed Abstract | CrossRef Full Text | Google Scholar
Vassey, J. K., Pawlowski, Thou., and Bergman, B. (2005). Root-based North2-fixing symbioses: Legumes, actinorhizal plants, Parasponia sp. and cycads. Found Soil 274, 51–78. doi: 10.1007/s11104-005-5881-five
CrossRef Full Text | Google Scholar
Walkley, A., and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic affair, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
CrossRef Full Text | Google Scholar
Widawati, Southward. (2015). Isolasi dan aktivitas plant growth promoting rhizobacteria (Rhizobium, Azospirillum, Azotobacter, Pseudomonas) dari tanah perkebunan Karet, Lampung [Isolation and activeness of institute-growth promoting Rhizobacteria (Rhizobium, Azospirillum, Azotobacter, Pseudomonas) from a rubber garden in Lampung]. Berita Biol. fourteen, 77–88.
Google Scholar
Yunita, C. A. Due east. (2016). Kajian Tekstur dan Porositas Tanah pada Daerah Terdampak Erupsi Gunung Kelud [Soil Texture and Porosity of Lands Affected past the Eruption of Mount Kelud]. Malang: Soil Science Department, Universitas Brawijaya.
Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/ffgc.2020.562303/full
0 Response to "What Causes Farmers to Return to the Slopes of Volcano to Begin Growing Crops Again"
Post a Comment